Tag Archives: pharmaceuticals
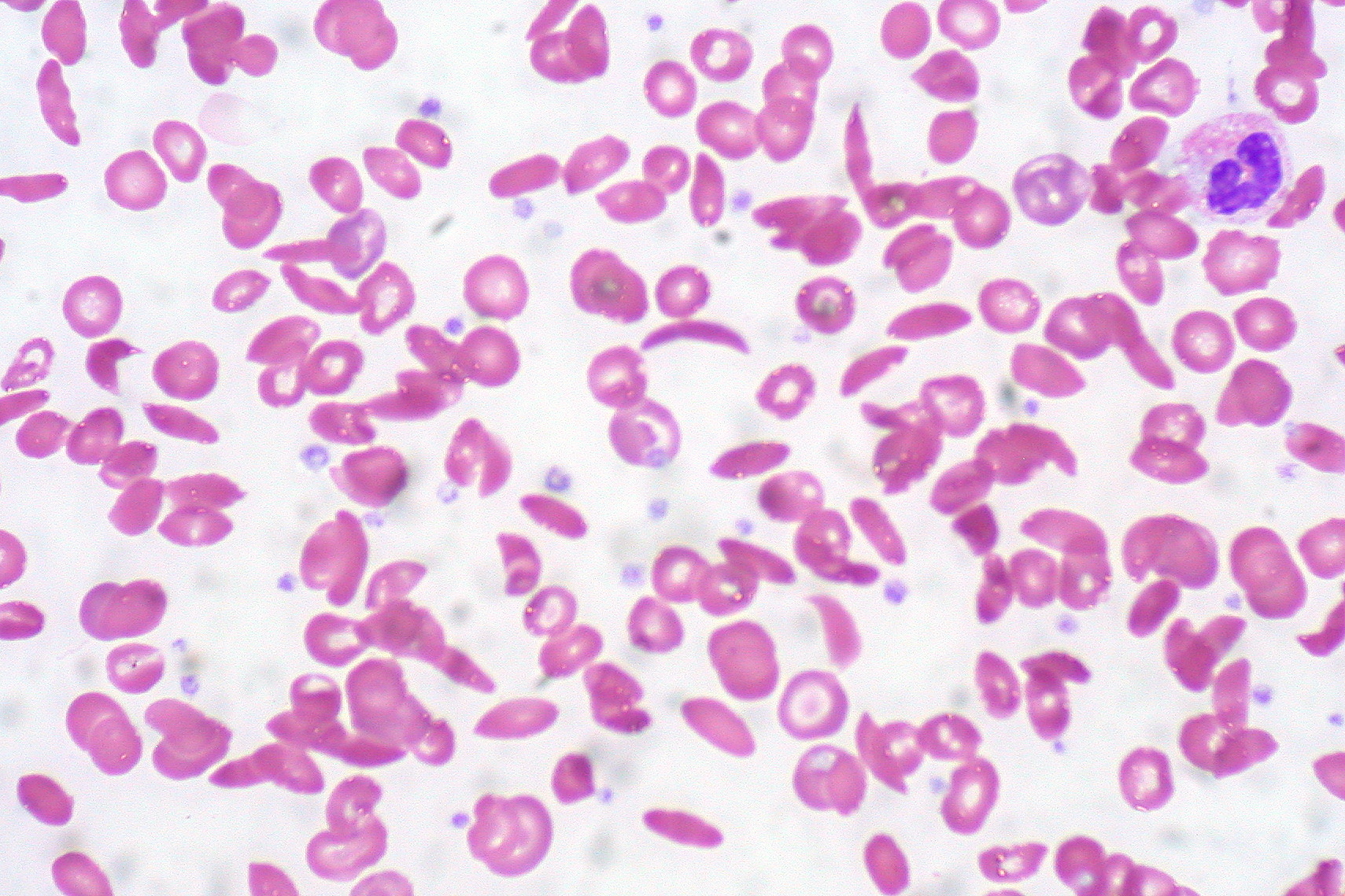
CASGEVY: Rethinking Drug Costs Through the Lens of Public Access
Maria Klimenko considers the extent to which the price of innovative treatments like CASGEVY, a type of gene therapy, should reflect not just the cost of development, but also their broader societal impact — and the public healthcare system’s ability to provide equitable access.

Whose Responsibility Are Ethical Issues in Phase 1 Research?
Dessislava Fessenko reveals that ethical concerns surrounding the participation of healthy volunteers in Phase 1 clinical trials have deep roots in broader social conditions, which require social policy responses.
Responding to the Opioid Epidemic
Matthew Herder considers possible regulatory responses to the opioid epidemic that aim to recover health care costs, compensate survivors, or prosecute pharmaceutical companies.
Clinical Trial Disaster in France
Jonathan Kimmelman comments on the recent phase 1 clinical research trial that left one healthy volunteer brain dead and five others hospitalized.
Enacting Pharmaceutical Transparency – Who, What, How, When, & Why
Matthew Herder shares a template letter that physicians, researchers, civil society groups, investigative journalists, and others can use to gain access to unpublished pharmaceutical safety and effectiveness data.



